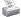I wasn't expecting people to post after me, so here's how I got the solution.

How I did this:
1. First, you've got to identify the unique compounds that don't have a partner. That is HCl, and Cl2. They are outlined with a rectangle.
2. Next, make everything even in number of mols. I did this by simply multiplying the top equation by 2.
3. You have got to have things you wish to eliminate by having them on opposite sides of the equation. Instead of switching the 1st and 2nd formula (which you should do), I made it easier to understand my solution by switching the 3rd one.
4. Eliminate common variables. 2NH3, 2NH4Cl, and N2 are all outright eliminated. There are 4 mols of H2 against 3 mols of H2, which leaves you with 1 mol of H2 on the right side of the equation.
5. Resulting equation is H2 + Cl2 --> 2HCl, but if you look at my equations above you realize I did it backwards. I'll address that in a second.
6. Resulting H is (-350) + (-99.22) + 628.86 = 179.64.
7. IMPORTANT, WHAT MANY PEOPLE DON'T EVEN THINK OF. Make sure you divide this by two, because you are only looking for ONE mol of HCl. This gives you 89.82.
8. I simply made it negative (or multiply by -1) because I did the equations earlier backwards. Result is -89.82
I hope that helps SilentWalker!



 !! Waiiit, you took chem, in commerce.. am I missing something? Please don't tell me that was your elective :/!
!! Waiiit, you took chem, in commerce.. am I missing something? Please don't tell me that was your elective :/!





 ) found it quite difficult...
) found it quite difficult...